
Pathogenicity and Survey of Root Rot Disease of Cotton in Different Villages of Dir Upper and Dir Lower Khyber Pakhtoon Khwa Pakistan
Shakir Ullah1, Lubna Shakir2and Muhammad Sajid3, Rihan Ullah3
1 State Key Laboratory of Systematic and Evolutionary Botany (LSEB), Institute of Botany Chines Academy of Science China
2 Department of Botany, Govt Degree College Timergara, Dir Lower Khyber Pakhtunkhwa Pakistan 3 Department of Botany, Bacha Khan University Charsadda, Khyber Pakhtunkhwa, Pakistan
| ARTICLE INFORMATION | ABSTRACT |
| Corresponding author: Shakir Ullah E-mail: Shakirawkum321@gmail.com Keywords: Disease incidence Rhizoctonia Cotton Dir Upper Dir Lower Kharif Received: 01.09.23 Received in revised form: 11.11.23 Accepted: 12.11.23 | The Survey was conducted for, the pathogenicity of root rot disease of cotton in different Village of Dir Lower and Dir Upper provoked by Rhizoctonia spp. 10 major cotton-growing Villages in Dir Upper and Dir Lower during Kharif 2019 and Kharif 2020. A roaming survey to record cotton root rot incidence was conducted in 10 major cotton-growing Villages of Dir Upper and Dir Lower during the months of July and August for consecutive two Kharif seasons 2019 and 2020. An average of 4 growing regions of cotton in each district was visited and the percent disease incidence was recorded. Among all the surveyed Village of Dir Upper, the maximum mean disease incidence was recorded in Samarbagh (18.75%) followed by Garrah (15.25 %), and Munda (15%), While the minimum disease incidence was recorded in Kabal and Charkhi Dadri 9.25% in both during kharif 2019. Among all the surveyed Village of Dir Upper, the maximum mean disease incidence was recorded in Samarbagh (18.25%) followed by Munda (15.25%), Garrah (15.42%), While the minimum disease incidence was recorded in Charkhi Dadri (9.08%). From the overall study, it can be understood that there is a prevalence of root rot disease in major mulberry growing locations in Dir Upper. Therefore, resistant cotton varieties are needed of the hour to address the grower’s problem along with best management strategies including efficient biocontrol agents which can minimize the disease to a greater extent. Therefore, a study should be undertaken to determine the disease prevalence in other locations. |
INTRODUCTION
The word ‘cotton’ refers to four species in the genus Gossypium (Family Malvaceae), namely G. hirsutum L., G. barbadense L., G. arboreum L. and G. herbaceum L. that are domesticated independently as sources of textile fiber. Globally, the Gossypium genus comprises about 50 species (Craven et al.1992).
During the year 2020-23, Pakistan is the 2nd largest country in world in terms of area under cotton is 133.50 lakh hectares which is 41% of the world cotton area. In terms of production, the country shares the leading position with China at 290 lakh bales of 480 lb (equivalent to 371 lakh bales of 170 kg). Cotton fiber is the purest source of cellulose and the most significant natural fiber.
The economic significance of cotton in the global market is evident by its majority share of over (50%) of fibers for textile goods (Anonymous et al. 2023). It is harvested as ‘seed cotton’ which is then ‘ginned’ to separate the seed and lint. The long ‘lint’ fiber is further processed by spinning to produce a yarn that is knitted or woven into fabrics. The ginned seed is covered in short, fuzzy fibers, Known as linters. Cotton is currently the leading plant fiber crop worldwide and is grown commercially in temperate and tropical regions of more than 50 countries (Chase et al. 1993).
Specific areas of production include countries such as the USA, India, China, the Middle East, and Australia, where climatic conditions suit the natural growth requirement of cotton, including periods of hot and dry weather, and where adequate moisture is available, often obtained through irrigation. The cotton crop is grown extensively with a limiting factor, that is infected by fungal diseases like anthracnose (Collectotrichum gossypii), leaf blight (Alternaria macrospora), wilt (Fusarium oxysporum f. sp. vasinfectum), Ramularia leaf spot (Ramularia areola), root rot (Rhizoctonia bataticola and Rhizoctonia solani) etc.
Out of all the diseases, root rot of cotton is the most devastating disease and nowadays this disease has become a major limiting factor in cotton cultivation. The management of root rot of cotton is of major concern wherever, cotton is cultivated thus, keeping in view the importance of the crop and destructive nature of disease, and the extent of losses it is causing to cotton cultivation in the state, the present investigation studies were undertaken.
Rhizoctonia is a widespread, destructive, and versatile plant pathogen, distributed worldwide in both agriculture and forest soils and is known to cause root diseases of several crop plants. Rhizoctonia bataticola (Taub.) Butler as a plant pathogen was recognized by Halsted. Taubenhaus gave the name of the genus Sclerotium because of the absence of spores and the species name as bataticola because it was pathogenic to Ipomea batatus (L.) Lam (Taubenhaus, 1913). According to the International Code of Botanical Nomenclature, the binomial Macrophomia phaseolin was the valid name for the pycnidia stage of R. bataticola.
Mycelia width varied from approximately 2-11µm and the distance between two consecutive septa measured 46µm. However, the most important character regarding taxonomy and classification was the production size and composition of microsclerotia the fungus produces root-like (rhizomorph) strands that grow through the soil until coming in contact with growing plant roots. Strands grow on roots toward the soil surface.
Immediately below the soil surface in cotton, the fungus proliferates around the hypocotyl, producing a cottony, mycelial growth. The bark is destroyed by this mycelium and the fungus fills the vascular tissue of the plant. Sclerotia form in the strands following the death of the plant. Sclerotia form from strands and the cells divide, grow and enlarge (Ullah et al. 2023).
These sclerotia are small (1/32 to 1/16 inch in diameter), densely compact masses of thick-walled cells. Sclerotia enables the fungus to persist in fallow soil or soil planted to resistant crops for several years. Sclerotia have been found up to 12 feet deep in some soils. Sclerotia within plant parts were black, smooth, hard and varied in size from 100 µm-1mm while in culture, it varied from 50-300µm. These descriptions were given by the Common Wealth Mycological Institute (CMI), Kew, England. During the sclerotia formation, 50–200 individual hyphal cells aggregate to give multicellular bodies called microsclerotia.
The microsclerotia were black and variable in size from 50–150μm depending on the available nutrients of the substrate on which the propagules were produced (Short, 1978). Symptoms are most likely to occur from August through September when soil temperatures reach 28°C (82 °F). The first symptoms are slight yellowing or bronzing of the leaves.
The uppermost leaves wilt within 24 to 48 hours after bronzing, followed by wilting of the lower leaves within 72 hours. Progressive wilting, premature dying, loss of vigor, and reduced yield are characteristic features of M. phaseolina infection. Permanent wilt occurs by the third day, followed by death. The leaves remain firmly attached to the plant.
Affected plants die suddenly, often after excellent growth. Trees and shrubs may die more slowly. Roots are usually extensively invaded by the fungus by the time wilting occurs. Affected plants can be pulled from the soil with little effort. The root bark is decayed and brownish, bronze-colored wooly strands of the fungus are frequently apparent on the root surface (Ullah et al. 2023). The fungus generally invades new areas by continual slow growth through the soil from plant to plant. It may also be moved about on roots of infected plants moved to new areas.
The fungus can survive in the soil for many years and often is found as deep in the soil as roots penetrate. Affected areas often appear as circular patterns of dead plants. These areas gradually enlarge during the season or in subsequent years as the fungus grows through the soil from plant to plant. Infested areas in cotton may increase by 5 to 30 feet per year in cotton (Yadav et al. 2017).
The pathogen M. phaseolina generally affects the fibrovascular system of the roots and basal internodes and impends the transport of nutrients and water to the upper parts of the plant. The disease actually starts much earlier and its above-ground manifestation in the form of wilting is a very late symptom.
The affected plants can easily be pulled out of the ground. The bark of roots is broken into shreds and gives a yellowish appearance as compared to healthy plants. Examination of affected parts reveals a dry rot, with many tiny black sclerotia distributed throughout the wood and softer tissues (Ullah et al. 2020). The aims and objectives of the research are to control the loss of crops and able the crops to survive in the environment. The main aim was to explore the possibility of the existence of different species and/or variables of root rot pathogen.
MATERIALS AND METHODS
A roving survey to record cotton root rot incidence was conducted in ten major growing Villages of Dir Upper and Dir Lower during the months of August and September for the consecutive two Kharif seasons 2019 and 2020. an average of 4 growing regions of cotton in each district were visited and the percent disease incidence was recorded by counting the total cotton plant in a 1 x 1m2 area and total root rot infected plants. Plants showing typical symptoms were also investigated for microscopic association of pathogen and final confirmation of pathogen by isolation, purification, and characterization. Typical symptoms like the straw-colored appearance of plants at pod formation, black rotted roots,
shredding of bark, and roots broken easily with the presence of minute dark black sclerotial bodies on root surfaces were considered for identification of disease. The percent disease incidence was calculated as per the formula given below
Survey for incidence and collection of disease samples
The diseased samples of cotton showing typical root rot symptoms were collected in Kharif 2019 and Kharif 2020 from farmer’s fields of different cotton growing areas of Dir Upper and Dir Lower viz., Garrah, Munda, Samarbagh, Mayyar, Timergara, Malakand, Maidan, Hall, Kabal, Chitral all from local landraces.
The main aim was to explore the possibility of the existence of the different species and/or variables of root rot pathogen, and incidence caused by them, and the survey was conducted in four villages selected randomly from each district and four fields from each village. The infected plants were carefully uprooted and placed in polythene bags, properly tagged and brought to the laboratory, and subjected to microscopic examination and tissue isolation.
Isolation, purification, and identification of pathogen
The pathogens were isolated on a potato dextrose agar (PDA) medium. Small pieces (1-2 mm) of diseased roots were cut, washed with sterilized water, surface sterilized with 0.1 percent sodium hypochlorite (NaOCl) solution for 1 minute followed by three to four washings with sterilized distilled water, and were transferred aseptically to 2 percent PDA (Potato Dextrose Agar) poured Petri plates.
The plates were incubated in an incubator at 28 ± 1 °C for 7 days. Hyphae coming out from the bits were subcultured on the fresh PDA in Petri dishes. From these bits mostly cultures of Rhizoctonia spp., Sclerotium spp., and Fusarium oxysporum were recovered. The culture of Rhizoctonia was purified by the single hyphal tip method. A total of 21 isolates of R. bataticola and R. solani in which 16 were R. bataticola and 5 were R. solani.
Pathogenicity test
The pathogenic ability of Rhizoctonia spp. was tested in a screen house on cotton cultivars HD 432 and RCH 773. Culture of Rhizoctonia was raised in a 250ml Erlenmeyer flask containing 50ml of PDB sterilized at 15 lbs. per sq inch pressure for 20 minutes. The bits of 5mm size were cut with the help of a sterilized cork borer from fresh pure culture plates (5 days old) and transferred into flasks with the help of a sterilized needle under aseptic conditions.
After 7 days of incubation in a BOD incubator at 27 ± 1⁰C, mycelial mats were collected and dried between folds of blotting paper for further studies. Five-gram fresh mycelial mat was homogenized in a blender for 2 minutes at the lowest speed in 1000ml of sterilized water. The suspension was used to inoculate the pots containing 10kg of sand: ground cotton seed mixture (9:1) which was sterilized by autoclaving at 15lbs/ inch pressure for one and half hours for two consecutive days.
On the third day of inoculation fifteen seeds of cotton cultivars, HD 432 and RCH 773 were sown in each plot. A separate set of un-inoculated pots was kept as control. Pots were irrigated regularly to maintain moisture. After 45 to 60 days of sowing, the symptoms appeared and the infected plants exhibited elongated lesions at the collar region which were later converted to dark brown to black and the stem was completely girdled by the lesions. The affected plants wilted, dried up later and can be uprooted easily. Diseased plants were brought to the laboratory and isolations were made on the PDA medium from diseased stem to confirm the identity of the pathogen.
RESULTS AND DISCUSSION
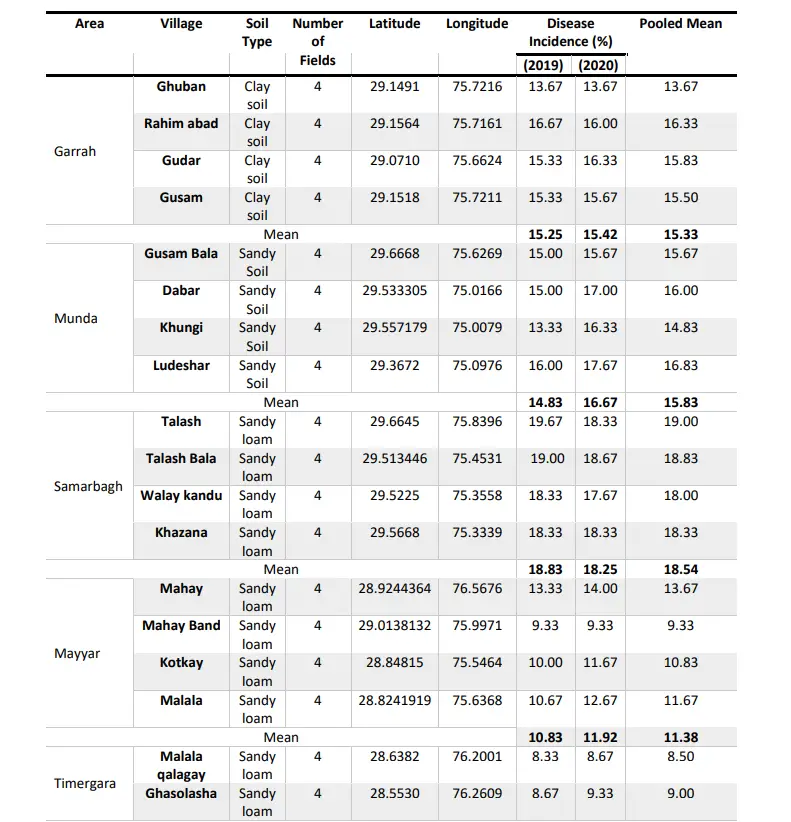
The intensive survey was carried out during Kharif, 2019 and 2020 in the cotton growing Village of Dir Upper viz., Garrah, Munda, Samarbagh, Mayyar, Charkhi Dadri, Malakand, Maidan, Hall, Kabal, Chitral to record the incidence of root rot in different Village. Cotton root rot incidence during Kharif, 2019 ranged from 7.67 to 19.67.
The data of Table 2 revealed that Talash village in Samarbagh district had the maximum disease incidence (19.67 %) followed by Talash Bala (19.00%) and 18.33% Walay Kandu and Khazana, whereas, the least disease incidence (7.67%) was recorded in village Sar Banda located in Kabal District. The results of the survey conducted in Garrah district showed that Rahim Abad had the highest disease incidence (16.67 %) followed by Gudar (15.33%) and Gusam (15.00%) and the minimum disease incidence was recorded in Ghuban village (14.00%).
In Munda district, the maximum disease incidence was recorded in Ludeshar (16 %) followed by Gusam Bala and Dabar (15.00%) while the minimum was recorded in Khungi (13.33 %). In Mayyar district, the maximum disease incidence was recorded in Mahay village (13.33%) followed by Malala (10.67%) and Kotkay (10.00%) while the minimum was recorded in Mahay Band (9.33%). In Charkhi Dadri district, the maximum disease incidence was recorded in Shukas (11.33%) followed by Ghasolasha and Sherhany (8.67%) while the minimum was recorded in Malala Calgary (8.33%).
In Mahendargarh district, the maximum disease incidence was recorded in Arrang (14.33%) followed by Narang (14 %) and Arrang bala (12.33%) while the minimum was recorded in Narang qala (10.67%). In Maidan district, the maximum disease incidence was recorded in Mamund Kotu (14.33%) followed by Bajaur (12.33%), and the least was recorded in both Qala Munda and Mamund (11.67%) In Hall district, the maximum disease incidence was recorded in Tnagay Har (12.33%) followed by Tnagay (11.33%) and Dandusha (9.67%) while the minimum was recorded in Shalkanday (9.33%).
In Kabal district, the maximum disease incidence was recorded in Laat (11.67%) followed by Maskeeny (9.00%) and Ghazi Baba (8.67%) while the minimum was recorded in Sar Banda (7.67%). In the Chitral district, the maximum disease incidence was recorded in Mangai (11.67%) followed by Mardan (11.33%) and the minimum was recorded in Sheen Ghar (10.33%) and the least was found in Swabay (10.00%). Among all the surveyed Village of Dir Upper the maximum mean disease incidence was recorded in Samarbagh (18.54%) followed by Munda (15.75%), Garrah (15.33%), Malakand (12.92 %), Maidan (12.75%), Mayyar (11.38%), Chitral (11.00%), Hall (10.54%) and Kabal (9.63%) While the minimum disease incidence was recorded in Charkhi Dadri 9.17% during kharif, 2019 depicted in Table 1.
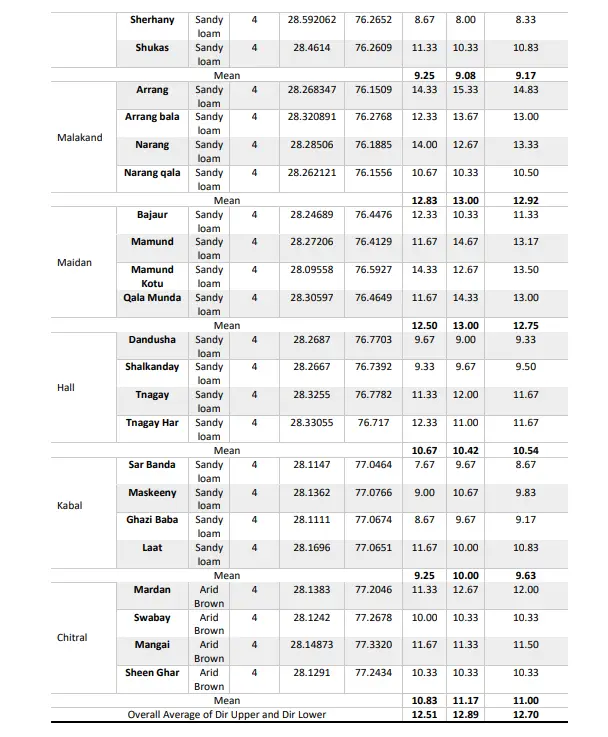
Cotton root rot incidence during Kharif 2020 ranged from 8.00 to 18.67. Among the surveyed villages in different Village of Dir Upper, Talash Bala village in Samarbagh district had the maximum disease incidence at 18.67% followed by Talash and Khazana both had 18.33% disease incidence in both villages and the least was in Walay Kandu (17.67%) whereas, overall least disease incidence was recorded in village Sherhany (8.00%) located in Charkhi Dadri district.
The results of the survey conducted in the Garrah district showed that village Gudar had the highest disease incidence (16.33 %) followed by Balsamanad (16.00%), Gusam (15.67 %) and the minimum disease incidence was recorded in Ghuban village (13.67 %). In Munda district, the maximum disease incidence was recorded in Ludeshar (17.67 %) followed by Dabar (17.00%) and Khungi (16.33%) while the minimum was recorded in Gusam Bala (15.67%).
In Mayyar district, the maximum disease incidence was recorded in Mahay (14.00%) followed by Malala (12.67%) and Kotkay (11.67%) while the minimum was recorded in Mahay Band (9.33%). In Charkhi dadri district, the maximum disease incidence was recorded in Shukas (10.33%) followed by Ghasolasha (9.33%) and Malala Calgary (8.67%) while the minimum was recorded in Sherhany (8.00%).
In Mahendargarh district, the maximum disease incidence was recorded in Arrang (15.33%) followed by Arrang bala (13.67%) and Narang (12.67%) while the minimum was recorded in Narang qala (10.33%). In Maidan district, the maximum disease incidence was recorded in Mamund (14.67%) followed by Qala Munda (14.33%) and Mamund Kotu (12.67%) while the minimum was recorded in Bajaur (10.33%). In Hall district, the maximum disease incidence was recorded in Tangay (12%) followed by Tnagay Har (11%) and Shalkanday (9.67%) while the minimum was recorded in Dandusha (9%).
In Kabal district, the maximum disease incidence was recorded in Ghaseda (10.67%) followed by Laat (10.00%) and the least was recorded both in Sar Banda and Ghazi Baba (9.67%). In the Chitral district, the maximum disease incidence was recorded in Mardan (12.67%) followed by Mangai (11.33%) and Dagay (10.33%) while the minimum was recorded in Sheen Ghar (10.33%).
Among all the surveyed Village of Dir Upper the maximum mean disease incidence was recorded in Samarbagh (18.25%) followed by Munda (16.67%), Garrah (15.42%), Malakand (13.00%), Maidan (13.00%), Mayyar (11.92%), Chitral (11.17%), Hall (10.42%), and Kabal (10.00%). While the minimum disease incidence was recorded in Charkhi Dadri (9.08%) as depicted in Table 2. Table 2 also revealed that the maximum average mean disease incidence during both Kharif, 2019 and Kharif, 2020 was recorded in Samarbagh district (18.54% and 18.25%) followed by Munda (15.75% and 16.67%) and the least was recorded in Charkhi Dadri (9.17%).
Table 1: Isolates collected from different Village of Dir Upper and Lower
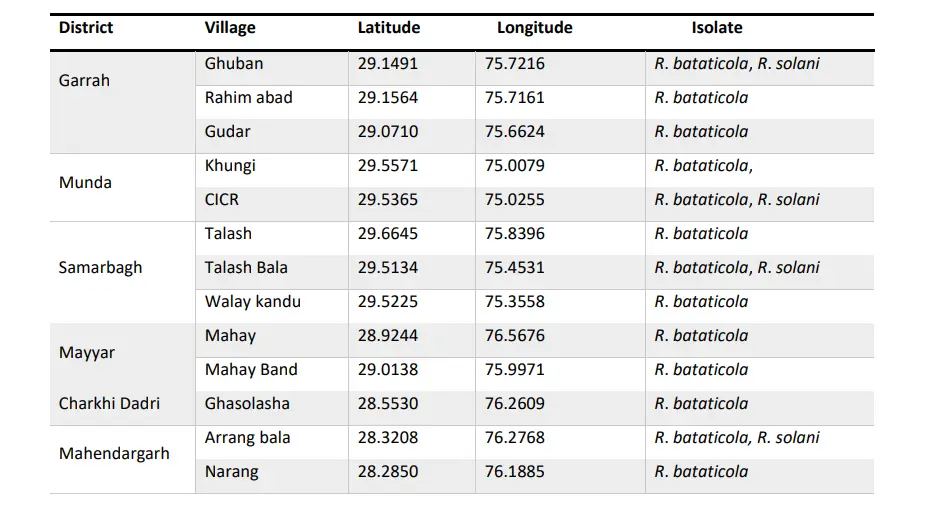
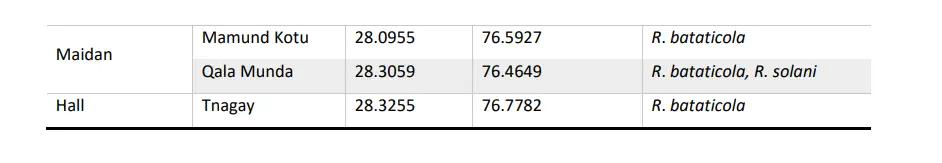
Table 2: Cotton root rot average disease incidence at different locations of Dir Upper during Kharif 2019 and Kharif 2020
CONCLUSION
The present study concluded that the incidence of root rot disease in cotton was detected in prominent cotton growing areas in Dir Upper. The highest average disease incidence was noticed in Samarbagh district during both Kharif 2019 and Kharif 2020 followed by Garrah district and the least average mean disease incidence was recorded in Charkhi Dadri and Kabal during Kharif 2019 and Charkhi Dadri during Kharif 2020. A total of 21 isolates of Rhizoctonia bataticola and Rhizoctonia solani were isolated from the infected root sample.
From the overall study it can be understood that there is a prevalence of root rot disease in major mulberry growing locations in Dir Upper. Therefore, resistant cotton varieties are needed of the hour to address the grower’s problem along with best management strategies including efficient biocontrol agents which can minimize the disease to a greater extent. Therefore, a study should be undertaken to determine the disease prevalence in other locations.
REFERENCES
Anonymous. Production of Crops. FAOSTAT, Food and Agriculture Organization of the United Nations. 2023
Bankoliya, M. K., Yadav, V. K., Khare, U. K., Amrate, P. K., Kumar, A., Sharma, R. C. Survey for dry root rot of chickpea caused by Rhizoctonia bataticola in a different region of Madhya Pradesh, India, 2022.
Chase, M. W., Soltis, D. E., Olmstead, R. G., Morgan, D., Les, D. H., Mishler, B. D., … & Albert, V. A. (1993). Phylogenetics of seed plants: an analysis of nucleotide sequences from the plastid gene rbcL. Annals of the Missouri Botanical Garden, 528-580.
Fryxell, P. A., Craven, L. A., McD, J. A revision of Gossypium sect. Grandicalyx (Malvaceae), including the description of six new species. Systematic Botany, 1992, 91-114.
Gordon, S., Hsieh, Y. L. (Eds.). Cotton: Science and technology. Woodhead Publishing. 2006. Khan, M. A., Khan, S. A., & Khan, R. W. Root rot disease complex of cotton: a menace to crop in Southern Punjab and its Mitigation through Antagonistic Fungi. Pakistan Journal of Zoology, 2017,49(5).
Lakhran, L., Ahir, R. R., Kumar, N., Nehra, D., Prajapati, S. Survey and occurrence of stem and root rot of sesame in different districts of Rajasthan. Phytochemistry and pharmacognosy, 2021.
Mohanapriya, R., Naveenkumar, R., Balabaskar, P. Survey, virulence and pathogenicity of root rot incidence of cowpea in selected districts of Tamilnadu caused by Macrophomina phaseolina (Tassi.) Goid. International Journal of Current Microbiology and Applied Science, 2017, 6, 694-705.
Mukunda, K., Teligi, V., Puttegowda, S. H., Sampangiramaiah, K. D. Disease incidence, severity and phenotypic variation among the isolates of Rhizoctonia bataticola infected in root rot disease of mulberry in different mulberry fields of Karnataka. Biosci Biotech Res Asia, 2021, 18(2), 403-411.
Short, G. E., Wyllie, T. D., Ammon, V. D. Quantitative enumeration of Macrophomina phaseolina in soybean tissues. Phytopathology, 1978, 68(5), 736-741.
Taubenhaus, J. J. The black rots of the sweet potato, International Journal of Current Microbiology and Applied Science, 1913, 9, 654-175.
Ullah, S., Ecological Study of Different Communities Site from District Lower Dir Laram Timergara Khyber Pakhtoon Khwa Pakistan. Journal of Botany, 2017. 1(1), 60-78.
Ullah, S., Shakir, L., Ullah, R. Morphological and Phytochemical Study of Cirsium arvense from District Mardan Pakistan. J Bioinfo Biotech Res, 2023, 1(1), 1-7.
Ullah, S., Ullah, R., Shakir, L., Ullah, R. Cheek list of ethno botanical plants of tehsil colony, Samarbagh, District Dir lower, Khyber Pakhtunkhwa Pakistan. Journal of Agriculture & Forestry Research.2023, 2-3.
Ullah, S., Ullah, Z., Iqbal, J., Abasi, F., Khan, S., Sohail, M., Ihsan, M. Traditional uses of plants and its role in the community development of sheen Ghar Valley district, Dir lower Khyber Pakhtunkhwa Pakistan. 2021,3,1; International Journal of Agriculture and Nutrition.
Yadav, R., Bunker, R. N., Sharma, S. S., Trivedi, A., Rawal, P. Survey, incidence and integrated disease management of cotton root rot caused by Rhizoctonia solani (Kuhn.). The Pharma Innovation Journal, 2022-11(8), 1618-1621.

