Morphometric Characterization and Length-length Relationship of Jagora spp. Collected from Tilapia Pond
Reyes, A. T.; De Dios, J. P.; Macasaquit, A. L.; dela Cruz, J. A. D.
College of Fisheries, Central Luzon State University, Science City of Muñoz, Nueva Ecija, Philippines
| ARTICLE INFORMATION | ABSTRACT |
| Article history: Received: 27.07.2021 Accepted: 06.09.2021 Published: *Corresponding author: E-mail: alvinreyes@clsu.edu.ph Keywords: Jagora Length-length relationship Morphometrics Snail | Fifty (50) pieces of Jagora spp. collected from tilapia pond in General Tinio, Nueva Ecija, Philippines were subjected to shell morphometrics in order to evaluate its length-length relationship. The shell morphometrics were presented as average: Shell length (SL) = 38.40±4.06 mm, Aperture length (AL) = 12.34±1.41 mm, Whorl height 1 (WH1) = 7.96±1.26 mm, Whorl height 2 (WH2) = 5.84±0.83 mm, Whorl height 3 (WH3) = 4.58±0.57 mm, Aperture width (AW) = 6.40±0.89 mm, Whorl width 1 (WW1) = 7.96±1.48 mm, Whorl width 2 (WW2) = 10.60±1.56 mm, Body whorl width (BWW) = 13.12±1.86 and Interior aperture length (AILL) = 8.52±1.00 mm. The correlations of SL to all of the considered morphometrics were strong or the r-value is from 0.6 to 0.8. The first three highest r values were recorded between SL-AL, SL-WH1, and SL-AW, thus, the value of AL, WH1, and AW could be best predicted given that SL is known. |
INTRODUCTION
Our country is considered a major ‘hot spot’ of species richness with a very high degree of endemism (Department of Environment and Natural Resources, 1997; Myers et al. 2000; Mittermeier et al., 2000). For example, a total of 519 vertebrate species are endemic to the Philippines, 64% of them are mammals (Heaney, 1998).
However, the documentation of the Philippine biodiversity is still continuing, in particular of invertebrate taxa. There is a scarcity of ecological, biogeographical, or evolutionary information for most representatives of the Philippine fauna and flora. At the same time, this rich biota is under severe threat due to the accelerating destruction of natural habitats (Dudgeon, 2000).
One of these limnetic groups is Cerithioidea, a basal caenogastropod superfamily with about 17 predominantly marine, but also some brackish and freshwater families. In approximation, this superfamily has 200 genera and several thousand species. This superfamily is of great ecological importance as grazers and detritus feeders in most tropical to subtropical aquatic ecosystems (Glaubrecht, 1996; Kohler and Glaubrecht, 2001).
One of the freshwater species under the superfamily Cerithioidea is Jagora spp. that is recorded in the northern part of the Philippines. It can grow up to 50 mm in length and 18 mm in width. Its shell is highly towered, usually dark brown in color. The body is gray to black with filiform antennae.
Jagora spp. is characterized by a unique reproductive system, including a long sperm gutter, a very short spermatophore bursa, and a prominent lateral ridge working as a seminal receptacle. Females carry eggs and juvenile stages within their mantle cavity. This snail feeds primarily on detritus and algae (Kohler and Glaubrecht, 2003; Kohler and Dames, 2009).
Morphological and morphometric studies in snails are important for species identification even in the advent of molecular tools (Prioli et al. 2002). Morphological measurements such as shell length, shell width, body whorl length, penultimate whorl width, aperture length, and aperture width are commonly used in the process of snail identification (Hamli et al. 2020).
The length-length relationship studies are practically used in the study of population dynamics, ecology, taxonomic differences, life history events, and stock management (Le Cren, 1951; Lagler et al. 1962; Abdoli et al. 2008; Ferreira et al. 2008; Vaslet et al. 2008; Epler et al. 2009).
This study aimed to morphologically characterize the shell of Jagora spp. that was collected from a tilapia pond in General Tinio, Nueva Ecija, Philippines and to eventually use these measurements to assess the length length relationship.
MATERIALS AND METHODS
Collection and preparation of samples
Fifty (50) pieces Jagora spp. were collected from a tilapia pond with 5 m in width and 10 m in length in General Tinio, Nueva Ecija. Snail samples were brought home for cleaning and visceral mass removal. The gastropod shell was photographed using a digital camera.
Measurement of shell
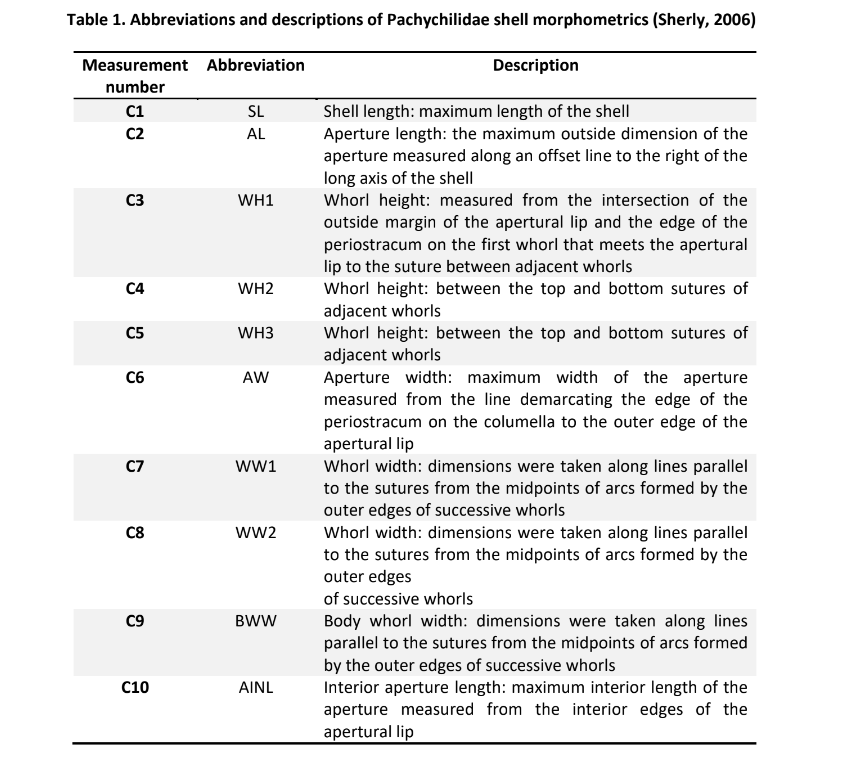
A total of 10 shell morphometrics were measured using a Vernier caliper based on the works of Sherely (1996) (Figure 1). The detailed shell morphometrics and characteristics are shown in Table 1.
Figure 1. Shell characteristics that are used in this study (Sherely, 1996)
Determinination of the degree of association between morphometrics
The degree of associations between the shell length (SL) and aperture length (AL), whorl heights (WHS), aperture width (AW), whorl widths (WWs), body whorl width (BWW), and interior aperture length (AINL) was based on the computed correlation coefficient (r) using trendline analysis in Microsoft (MS) Excel.
Estimation of length-length relationship
The length-length relationship of the snail samples was estimated using the formula: X = ea(SLb).
Where:
SL = shell length in mm
W = the other length in mm (AL, WH, AW, WW, BWW, and AINL)
a = intercept
b = slope
Table 1. Abbreviations and descriptions of Pachychilidae shell morphometrics (Sherly, 2006)
Measurement number
Abbreviation Description
C1 SL Shell length: maximum length of the shell C2 AL Aperture length: the maximum outside dimension of the aperture measured along an offset line to the right of the
long axis of the shell
C3 WH1 Whorl height: measured from the intersection of the outside margin of the apertural lip and the edge of the
periostracum on the first whorl that meets the apertural
lip to the suture between adjacent whorls
C4 WH2 Whorl height: between the top and bottom sutures of adjacent whorls
C5 WH3 Whorl height: between the top and bottom sutures of adjacent whorls
C6 AW Aperture width: maximum width of the aperture measured from the line demarcating the edge of the
periostracum on the columella to the outer edge of the
apertural lip
C7 WW1 Whorl width: dimensions were taken along lines parallel to the sutures from the midpoints of arcs formed by the
outer edges of successive whorls
C8 WW2 Whorl width: dimensions were taken along lines parallel to the sutures from the midpoints of arcs formed by the
outer edges
of successive whorls
C9 BWW Body whorl width: dimensions were taken along lines parallel to the sutures from the midpoints of arcs formed
by the outer edges of successive whorls
C10 AINL Interior aperture length: maximum interior length of the aperture measured from the interior edges of the apertural lip
RESULTS AND DISCUSSION
Shell morphometrics
In Table 2, the shell measurements of 50 pieces of Jagora spp that were collected in a tilapia pond in General Tinio, Nueva Ecija, Philippines are presented. Ten (10) shell measurements were considered in this study, namely shell length (SL), aperture length (AL), whorl height 1 (WH1), whorl height 2 (WH2), whorl height 3 (WH3), aperture width (AW), whorl width 1 (WW1), whorl width 2 (WW2), body whorl width (BWW) and interior aperture length (AILL).
Table 2. Average shell measurements of Jagora spp. that were collected in tilapia a pond in Gneral Tinio, Nueva Ecija, Philippines
| Shell morphometrics | Average measurement (mm) |
| Shell Length (SL) | 38.40±4.06 |
| Aperture Length (AL) | 12.34±1.41 |
| Whorl Height 1 (WH1) | 7.96±1.26 |
| Whorl Height 2 (WH2) | 5.84±0.83 |
| Whorl Height 3 (WH3) | 4.58±0.57 |
| Aperture Width (AW) | 6.40±0.89 |
| Whorl Width 1 (WW1) | 7.96±1.48 |
| Whorl Width 2 (WW2) | 10.60±1.56 |
| Body Whorl Width (BWW) | 13.12±1.86 |
| Interior Aperture Length (AILL) | 8.52±1.00 |
The snail SL ranged from 30 to 48 mm (38.40±4.06 mm) with 35 and 40 mm as the most common. AL was from 10 to 18 mm (12.34±1.41 mm) with 12 mm as the most frequent. WH1 was from 6 to 10 mm (7.96±1.26 mm) with 9 mm as the dominant. WH2 fluctuated from 4 to 8 mm (5.84±0.83 mm) with 6 mm as the foremost. WH3 ranged from 4 to 6 mm (4.58±0.57 mm) with 4 mm as the most frequent.
AW was recorded from 5 to 8 mm (6.40±0.89 mm) with 6 mm as the dominant width. WW1 ranged from 4 to 12 mm (7.96±1.48 mm) with 7 mm as the leading width. WW2 ranged from 5 to 14 mm (10.60±1.56 mm) with a width of 10 mm as the most common. BWW was from 7 to 19 mm (13.12±1.86 mm) with 12 mm width as the most frequent. AILL varied from 7 to 11 mm (8.52±1.00 mm) with 9 mm as the most dominant (Table 2).
According to several studies, the shell measurements are influenced by ecological factors such as latitude, depth of distribution, tidal excursion or shore level, water movements such as waves, turbulence and currents, type of sediment, and trophic conditions (Fiori and Defeo, 2006; Claxton et al. 1998; Franz, 1993; Akester and Martel, 2000; Nagarajana et al. 2006). In Jagora spp., there are no available morphometrics studies on the various factors that might influence shell morphometrics. This present study only provides basic information on the shell morphometry of Jagora spp. that was only collected from a tilapia pond.
Degree of association between shell length and the rest of shell morphometrics
A correlation coefficient measures the statistical relationship between two variables: the correlation between SL and the rest of the shell morphometrics. The correlations of SL to all of the morphometrics were strong or r value is from 0.6 to 0.8 (SL AL = 0.785, SL-WH1 = 0.782, SL-WH2 = 0.628, SL-WH3 = 0.605, SL-AW = 0.769, SL-WW1 = 0.756, SL-WW2 = 0.699, SL-BWW = 0.653 and SL-AILL = 0.741).
All paired variables showed direct relationship as indicated by positive b values (SL-AL = 0.803, SL-WH1 = 0.211, SL WH2 = 0.855, SL-WH3 = 0.698, SL-AW = 1.017, SL-WW1 = 1.370, SL-WW2 = 1.069, SL BWW = 0.895 and SL-AILL = 0.815), thus, an increase of 1 unit in the X variable will result to a certain unit of increase in the Y variable (Table 3).
The first three highest r values were recorded between SL-AL, SL-WH1, and SL-AW, thus, the value of AL, WH1, and AW could be best predicted given that SL is known. The computed values of r and b in this present study are impossible to compare because of the unavailability of literature about Jagora spp.
Table 3. Intercept (a), slope (b), coefficient of determination (r2), and correlation coefficient (r) of the paired shell morphometrics.
| Paired variables | a | b | r2 | r |
| SL-AL | -0.182 | 0.803 | 0.617 | 0.785 |
| SL-WH1 | -0.030 | 0.211 | 0.612 | 0.782 |
| SL-WH2 | -0.590 | 0.855 | 0.394 | 0.628 |
| SL-WH3 | -0.447 | 0.698 | 0.366 | 0.605 |
| SL-AW | -0.807 | 1.017 | 0.592 | 0.769 |
| SL-WW1 | -1.274 | 1.370 | 0.571 | 0.756 |
| SL-WW2 | -0.671 | 1.069 | 0.489 | 0.699 |
| SL-BWW | -0.302 | 0.895 | 0.427 | 0.653 |
| SL-AILL | -0.361 | 0.815 | 0.549 | 0.741 |
Length-length relationship equation
In Table 4, the summary of length-length equations is provided. These equations could be used to predict the value of AL (0.834 SL0.803), WH1 (0.970 SL0.211), WH2 (0.554 SL0.855), WH3 (0.640 SL0.698), AW (0.446 SL1.017), WW1 (0.280 SL1.370), WW2 (0.511 SL1.069), BWW (0.739 SL0.895) and AILL (0.697 SL0.815) if SL is given. The first three highest r value was recorded in pairs SL-AL, SL-WH1, and SL-AW. The values of AL, WH1, and AW could be best predicted given that SL is known.
The length-length relationship studies are practically used in the study of population dynamics, ecology, taxonomic differences, life history events, and stock management (Le Cren, 1951; Lagler et al. 1962; Abdoli et al. 2008; Ferreira et al. 2008; Vaslet et al. 2008; Epler et al. 2009).
Table 4. Length-length relationship (LLR) equation of the paired shell morphometrics.
| Paired variables | LLR equation |
| SL-AL | AL = 0.834 SL0.803 |
| SL-WH1 | WH1 = 0.970 SL0.211 |
| SL-WH2 | WH2 = 0.554 SL0.855 |
| SL-WH3 | WH3 = 0.640 SL0.698 |
| SL-AW | AW = 0.446 SL1.017 |
| SL-WW1 | WW1 = 0.280 SL1.370 |
| SL-WW2 | WW2 = 0.511 SL1.069 |
| SL-BWW | BWW = 0.739 SL0.895 |
| SL-AILL | AILL = 0.697 SL0.815 |
CONCLUSION
The correlations of SL to all the morphometrics were strong or the r value is from 0.6 to 0.8. The first three highest r values were recorded between SL-AL, SL WH1, and SL-AW, thus, the value of AL, WH1, and AW could be best predicted provided that SL is known.
The following were recommended for the improvement of future studies: (1) compare the shell morphometrics and LLR equation of Jagora spp. collected from ponds, streams and rivers, and irrigation canals; (2) consider the influence of sex in the shell morphometrics and LLR equation of Jagora spp.; and (3) increase the frequency of sampling.
REFERENCES
Abdoli, A.; Rasooli, P.; Mostafavi, H. Length weight relationships of Capoeta capoeta capoeta (Guldenstaedt, 1772) in the Gorganrud River, South Caspian Basin. Journal of Applied Ichthyology. 2009, 24, 96-98.
Akester, R.; Martel, A. Shell shape, dysodont tooth morphology, and hinge-ligament thickness in the bay mussel Mytilus trossulus correlate with wave exposure. Canadian Journal of Zoology. 2020, 78(2), 240-253.
Department of Environment and Natural Resources. Philippine biodiversity: An assessment and action plan. United Nations Environment Programme. Makati City: Bookmark, Inc., 1997, 1-40 pp.
Dudgeon, D. Ecological strategies of Hong Kong Thiaridae (Gastropoda: Prosobranchia). Malacological Review. 2020, 22, 39-53.
Epler, P.; Nowak, M.; Popek, W. Growth rate of the chub (Squalius cephalus) and the nase (Chondrostoma nasus) from Raba, Dunajec and Poprad river. AACL Bioflux. 2009, 2, 1-8.
Franz, D. R. Allometry of shell and body weight in relation to shore level in the intertidal bivalves Geukensia demissa (Bivalvia: Mytilidae). Journal of Experimental Marine Biological and Ecological. 1993, 174(2), 193-207.
Ferreira, S.; Sousa, R.; Delgado, J.; Carvalho D.; Chada, T. Weight-length relationships for demersal fish species caught off the Madeira archipelago (eastern-central Atlantic). Journal of Applied Ichthyology. 2008, 24, 93-95.
Fiori, S.; Defeo, O. Biogeographic patterns in life-history traits of the yellow clam, Mesodesma mactroides, in Sandy Beaches of South America. Journal of Coastal Research. 2006, 224, 872-880.
Glaubrecht, M. Evolutionsökologie und Systematik am Beispiel von Süß- und Brackwasserschnecken (Mollusca: Caenogastropoda: Cerithioidea): Ontogenese-Strategien, paläontologische Befunde und historische Zoogeographie. Leiden: Backhuys, 1996, 1-230 pp.
Hamli, H.; Hamed, N. A.; Azmai, S. H. S.; Idris, M. H. Conchology variations in species identification of Pachychilidae (Mollusca, Gastropoda, Cerithiodea) through multivariate analysis. Tropical Life Sciences Research. 2020, 31(2), 145-158.
Heaney, L. R. A. Synopsis of the mammalian fauna of the Philippine Islands. Fieldiana: Zoology. New Series. 1998, 28, 1-61.
Köhler, F.; Dames, C. Phylogeny and systematics of the Pachychilidae of mainland Southeast Asia – Novel insights from morphology and mitochondrial DNA (Mollusca, Caenogastropoda, Cerithioidea). Zoological Journal of the Linnean Society. 2009, 157, 679-699.
Köhler, F.; Glaubrecht, M. Morphology, reproductive biology and molecular genetics of ovoviviparous freshwater gastropods (Cerithioidea: Pachychilidae) from the Philippines, with description of the new genus Jagora. Zoologica Scripta. 2003, 32(1), 35-59.
Köhler, F.; Glaubrecht, M. Toward a systematic revision of the Southeast Asian freshwater gastropod Brotia H. Adams, 1866 (Cerithioidea:Pachychilidae): An account of species from around the South China Sea. Journal of Molluscan Studies. 2001, 67, 281-318.
Lagler, K. F.; Bardach, J. E.; Miller, R. R. Ichthyology. John Wiley and Sons, Inc., New York, 1962, 1-546 pp.
Le Cren, E. D. The length-weight relationships and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). Journal of Animal Ecology. 1951, 20, 201- 219.
Mittermeier, R. A.; Myers, N.; Mittermeier, C. G. Hotspots: Earth’s biologically richest and most endangered terrestrial ecoregions. Chicago: University of Chicago Press, 2000, 1-345 pp.
Myers, N.; Mittermeier, R. A.; Mittermeier, C. G.; da Fonseca, G. A. B.; Kent, J. Chicago: Biodiversity hotspots for conservation priorities. Nature. 2000, 403, 853-858.
Nagarajana, R.; Lea, S.; Goss-Custard, J. Seasonal variations in mussel, Mytilus edulis L. shell thickness and strength and their ecological implications. Journal of Experimental Marine Biology and Ecology. 2006, 339(2), 241-250.
Prioli, S. M.; Prioli, A. J.; Julio, H. F.; Pavanelli, C. S.; Oliveira, A. V.; Carrer, H.; Prioli, L. M. Identification of Astyanax altiparanae (Teleostei, Characidae) in the Iguaçu. Genetic and Molecular Biology. 2002, 25(4), 421-430.
Sherely, G. Morphological variation in the shells of Placostylus species (Gastropoda: Bulimulidae) in New Zealand and implications for their conservation. New Zealand. Journal of Zoology. 1996, 23(1), 73-82.
Vaslet, A.; Bouchon-Navaro, Y.; Louis, M.; Bouchon, C. Weight-length relationships for 20 species collected in the mangroves of Guadeloupe (Lesser Antiles). Journal of Applied Ichthyology. 2008, 24, 99-100.

